A Return to the Beginning
An honest examination of what I am doing, and why I am here.
The Beginning
In September of 2021, I went to visit my aunt, Kathleen Carlotti for dinner on a cool autumn evening. “Aunty Kathy,” as we affectionately called her, had the usual spring in her step that we had all known her so well for. Her favorite songs filled the house as she danced between the stove and the pantry, calling out orders for us to set the table. She was vibrant, weaving throughout the kitchen as if she was skiing down the slopes (she was an adrenaline chaser at heart). She had a persistent enjoyment of life that made her presence in mine so unforgettable.
Just seven months later, in April of 2022, I visited my aunt again. This time, the house held a saddening silence. No music, no footsteps on the kitchen tiles, just the hum of the motorized wheelchair she was confined to. By this point, she was unable to walk, and barely able to open her own hands. Behind her eyes, she was still there, but her body was no longer capable of keeping up with the wit of her mind. Her humor carried on in the slowed sentences that grew more difficult for her to say as time marched on.
The only difference that took hold in those seven months, was the rapid degeneration of motor neurons at the hands of amyotrophic lateral sclerosis (ALS). An age-related neurodegenerative disease, ALS stole her ability to walk, to talk, to ski, to dance, and eventually, to live. Long before she took her last labored breath, the atrophy of those motor neurons stole so much of the life she had left to live.
The neurons were there, then they were gone… and within less than a year, so was my aunt.
—
Roughly a year later, I peered through a microscope at Boston University’s Brain Bank, flipping through stained brain sections of a patient who had ALS, trying to understand what happened and why. This started me down a path of attempting to understand the loss of those neurons in many forms: from the degenerative condition my aunt underwent, to the several concussions I suffered, and beyond. For my whole life, I thought I would be pursuing public service, helping enhance education systems at home and abroad; I never thought I would be spending a cold January night in my sophomore year of college staring at the same disease that killed my aunt. I never thought I would be spending my summers in a dark basement, scanning through images of mouse brains subjected to repetitive traumatic brain injuries. I never imagined holding the severely damaged brain of a patient with suspected chronic traumatic encephalopathy (CTE), or growing cortical organoids for drug discovery efforts to reverse ALS in the dish. I was never supposed to be doing any of this. Yet, as much as I wanted to know why we didn’t have cures for any of these (let alone why they occurred in the first place) I also wondered if I, of all people, could figure it out.
Every time I tried to walk away from that cliff – after countless approaches driven by sorrow, rage, and helplessness – one thing pushed me back to the edge time and time again: in every disease state I studied, every mouse section I stained, every brain I held: there were neurons there, and then they were gone. Atrophy was everywhere I looked, and I couldn’t seem to escape it. So I took the leap, leaving behind what I had built and picking a fight I didn’t even know my odds of winning.
The Dogma
All neurodegenerative diseases (NDDs) share a common thread, implied in the name: the loss of neurons.1 While there are many etiologies for how pathology, inflammation, toxicity, or other mechanisms lead to that manifestation, the result is the same. Furthermore, it is worth acknowledging that many of the phenotypes we recognize as “disease” often occur once the neurons – and the function they provided – are gone, not from the various developments prior.
Thinking about the most common NDDs, this atrophy of neurons takes hold among different subtypes in different regions of the brain, yielding different outcomes. In ALS, it’s motor neurons; in Alzheimer’s it’s cortical and hippocampal neurons; and in Parkinson’s, it’s dopaminergic neurons. These three diseases are emblematic of the negative effects of neuronal loss, while countless other causes of neurodegeneration continue to plague the minds of millions each year.
Atrophy is a real consequence of age-related neurodegenerative diseases (and other conditions such as stroke, traumatic brain injuries, and damage-associated diseases like CTE). Yet, tragically, we have no great answer for this other than trying to find the needle(s) in the haystack that might prevent it. There are essentially two camps focusing on how to address this issue:
Prevent the loss of those neurons, or
Replace the neurons that are lost.
I used to be a member of the first camp, and still think research into preventing and slowing disease is very important. However, we only vaguely understand why these diseases really start with no unifying approach to tackle their various etiologies. As a result, neurologists around the world are stuck playing whack-a-mole trying to beat back pathologies without getting to either the root of the problem or a cure. That goes without acknowledging the inherent stochasticity that is very difficult to address in cases of neuronal loss from injury or non-disease related atrophy (including in general brain aging).
My aunt’s care team faced this same predicament, providing approved medications, enrolling her in clinical trials, and more… all to no avail. The hope of slowing the disease did nothing for her, as is the case for the thousands of others who were diagnosed with ALS that year (and millions of others with Alzheimer’s and Parkinson’s). There are no real treatment plans, only end of life plans.
For centuries we now, we have been held helpless to intervene at the level of neuronal loss due to a dogma of neuroscience that has been on my mind since watching my own aunt pass away from a disease of neuronal loss:
“Everything in the brain may die… nothing may be regenerated. It is for the science of the future to change, if possible, this harsh decree” – Santiago Ramon y Cajal, 1906
Ramon y Cajal, the father of modern neuroscience, was certainly right in two regards here: first, the brain does not, on its own, have the capacity to meaningfully regenerate new neurons or connections – at least not in any way that can compensate for the scale of loss we see in disease or injury.2 Second, his “science of the future” is now the science of today and we have the capability to “change, if possible, this harsh decree.”
The Circuit
For just a moment, consider a light bulb. Over time, when the filament wears out, a good bulb grows dimmer, or eventually burns out entirely. Do we (and should we) use the lightbulb less to prevent it from burning out? How about replacing individual parts like a filament or the casing? Or should we just do away with lightbulbs entirely? No, we just replace the whole bulbs with newer, better ones.
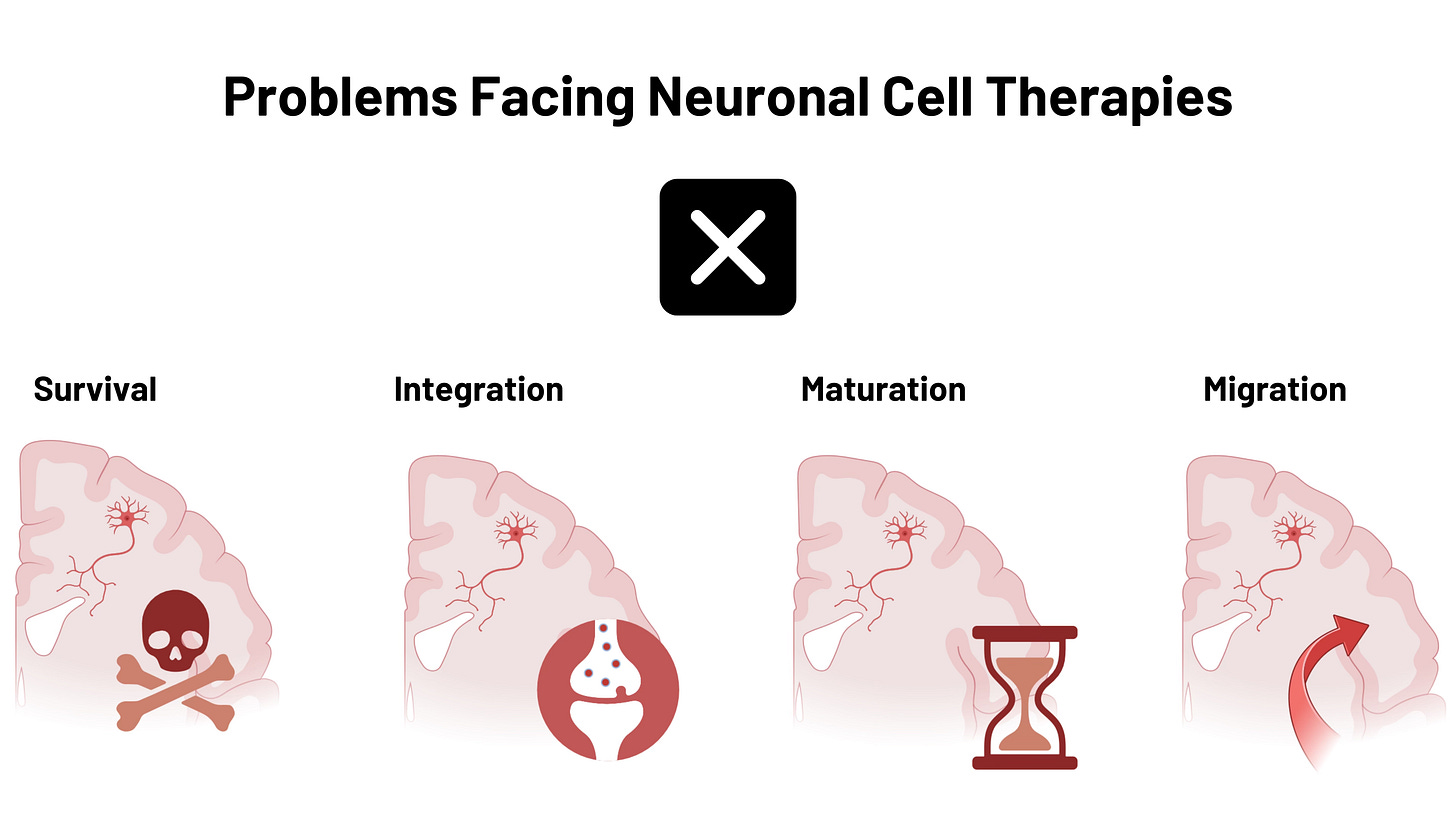
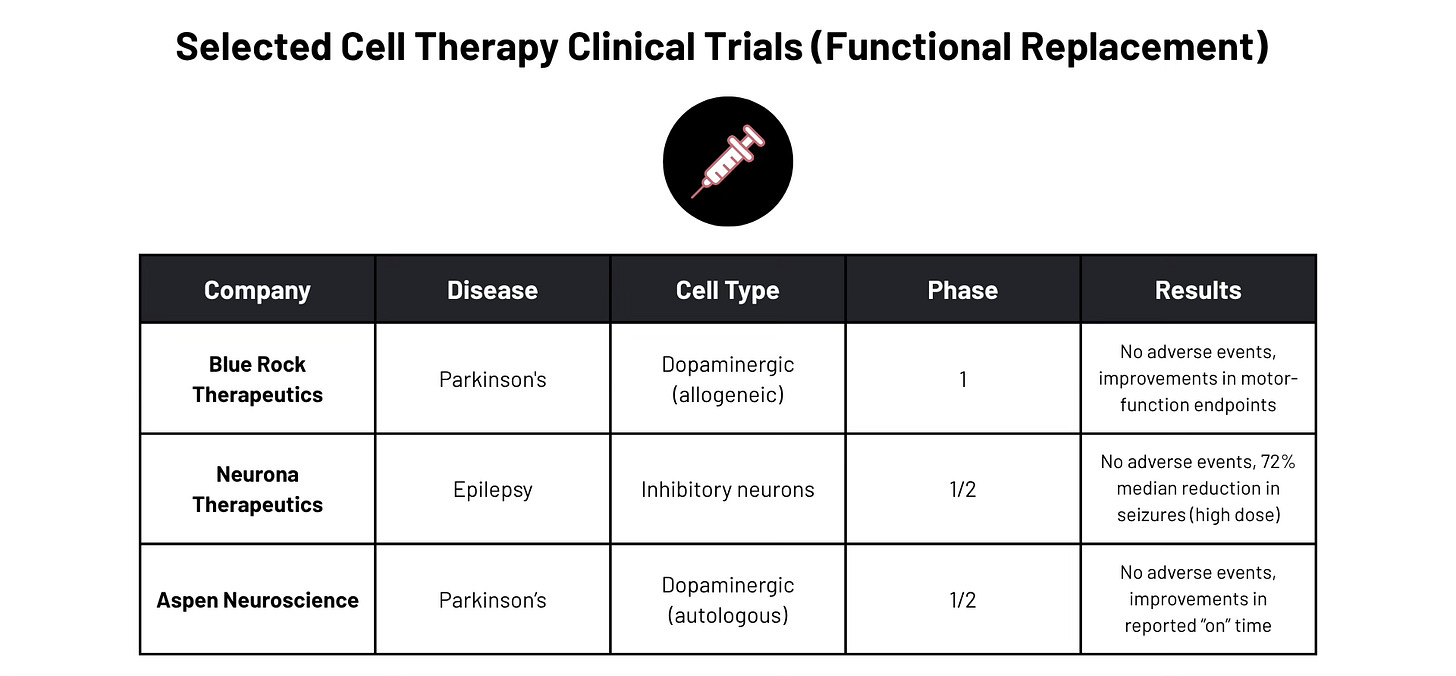
What if we approached diseases of neuronal loss in much the same fashion? What if we replaced neurons that are dead with new ones. Looking at decades of preclinical research and recent clinical success in the Parkinson’s and epilepsy space, what if we could build better neurons that are capable of restoring lost function by integrating into existing neural circuits? How could we enhance the differentiation, the resilience, the axonal guidance, the maturation, and other metrics of “success” in a neuronal replacement?
There is much engineering to be done to get us to a point where we can, with great precision, safety, and efficacy, functionally restore lost circuits. But just because it is hard, does not mean it should not – or cannot – be done. Hell, we put a man on the moon in the same century we learned how to take flight… what says we can’t conquer this formidable frontier in our lifetimes too?
What if, for my aunt, after 7 months of failing to slow the disease’s progression, we could rebuild the motor circuits that allowed her to move and make them more resilient? Imagine if we could re-innervate the muscles that allowed her to speak, to ski, and ultimately, to live. Imagine if, instead of well wishes, we could offer a second chance at life by replacing what is lost.
Replacement is what we can and must do when prevention fails. But despite our efforts to make better drugs, target new pathways, and better understand the genetics, we sadly still know so little about how these diseases progress other than one simple fact: the neurons were there, then they weren’t.
Even if we were able to find the ultimate pause button for these diseases (which, for ALS has been sadly elusive3), it is unlikely we could prevent every injury or stroke, and even less realistic to shield ourselves from the inherent stochasticity that underlies non-disease related brain aging and atrophy. Even if we were able to develop the perfect drug, for so many people with Parkinson’s and ALS the damage is done long before a diagnosis comes through (roughly 80% of the dopaminergic neurons and 33-50% of the motor neurons, respectively, are lost by the time a diagnosis is reached). While I’d welcome better early detection methods, I’d be even more enthusiastic about a means of reversing the loss of those 50-80% of neurons. The good news? These are not mutually exclusive – through neuronal transplantation preclinical studies (and even the earliest in human trials) we have learned a great deal about the mechanisms by which these diseases destroy cells, and lives.
The Fight
As of writing this, I want to emphasize that I might not know exactly how or when that harsh decree will be defeated, but that I will do everything I can to end its reign. I lost someone I loved to neuronal loss, and I’ve heard countless stories of how this failure of human biology has taken far too many lives. It kills indiscriminately like a serpent poisoning the fruit of life, as a punishment for no known sin. When I think about what it was like for my aunt to be on her deathbed, trapped inside the lifeless cage that had become of her body, I want to fight with everything I’ve got.
I don’t know how this journey is going to end or if it ever will in my lifetime, but as someone who picked up his first pipette in search of revenge, I’m going back to the start in the hope of finding some clarity. This piece is, in a way, a means of coming to terms with the enormity of this fight and my undying conviction to win it, as a person who left behind a life of public service to bring an end to an enemy we still don’t fully understand. This is a return to the beginning… an honest examination of what I am doing and why I am here.
Even if we don’t have the exact answers on how to overcome the engineering barriers that face cell therapies in the brain as of now, there are many promising tools in the kit and techniques that could be employed (gene editing, optogenetics, connectomics, etc.). Maybe it isn’t about tailoring the environment to the cells, but vice versa: equipping these cells with what they need to accomplish the incredible task we are asking of them. Maybe this requires better means of measuring the “success” of these cells as well as large-scale, open source, efforts to enable better replacement techniques across cell types.
All said, what I do have, is a vision of a world where neuronal loss is not an immutable fact of life, but something that can be easily reversed for large swaths of the population. I have a vision of a world where my aunt’s diagnosis was not one of a terminal nature, but of a temporary illness that could be overcome; where a doctor would tell her how many months it would be until she could dance in the kitchen again or ski on the slopes, rather than being told how many months it would be until she was buried in her grave.
Onward
If you’re reading this, and want to join in on the fight, I would love to hear from you and to talk more (email seanrsimonini[at]gmail[dot]com). If you have ideas on how to better engineer cells to restore lost function via transplantation into the brain, I am all ears. I am open to hosting events, journal clubs, co-working sessions, coffee chats, or really anything to bring together the right people for solving this problem. I will also be writing more on this in the future, specifically on my thoughts of the “what now?” and what I think are the most promising avenues to win this fight.
Let’s bring the science of the future to life and change this harsh decree, together.
Yes, there are NDD’s that are in the minority of cases where neuronal function (such as via synaptic degradation) is impacted but cell death is largely absent (eg: minimal atrophy AD). While I am specifically focusing on the death of neurons here, I acknowledge those cases and emphasize that replacement approaches are worth researching in those circumstances as well. Whether this could extend to neuropsychiatric conditions where neuronal dysfunction rather than atrophy is largely at play, remains to be seen.
Yes, there is synaptic plasticity and (likely, although hotly debated) some limited endogenous neurogenesis in the adult, mammalian dentate gyrus and SVZ. However, this is greatly limited by small pools of non-quiescent neural stem cells available to form new neurons and the fact that spontaneous regeneration of large portions of neural tissue is not seen in the adult mammalian brain.
There are currently only 4 FDA-approved interventions for ALS (with one being a gene therapy targeted to a familial variant known as SOD1). The remaining three for the >90% of patients with sporadic ALS only marginally improve outcomes (on a timescale of months) or merely manage symptoms. Numerous efforts to target various mechanisms of the disease have failed, possibly due to how little is known about the disease, its causes, or its mechanisms of action.